Jumat, 12 Juni 2020
HUMAN ACTIVITY IS DISSOLVING THE OCEAN FLOOR
High degrees of human-made CO2 causes sea acidification that's quickly dissolving the seafloor, a brand-new study cautions.
Normally the deep sea bottom is a milky white. It is made up, to a large degree, of the mineral calcite (CaCO3) formed from the skeletons and coverings of many planktonic microorganisms and corals reefs.
The seafloor plays a crucial role in managing the level of sea acidification. The dissolution of calcite reduces the effects of the acidity of the CO2, and at the same time prevents seawater from ending up being too acidic.
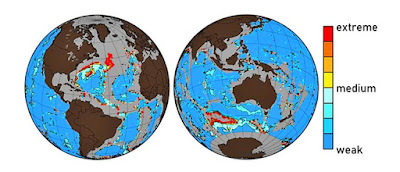
A map showing locations of the seafloor that sea acidification has affected, to differing levels. (Credit: McGill)
But nowadays, at the very least in certain hotspots such as the north Atlantic and the southerly seas, the ocean's milky bed is ending up being more of a murky brownish. Because of human task, the degree of CO2 in the sprinkle so high—and the sprinkle is so acidic—that the calcite is simply dissolving.
FATED FUTURE
Scientists say they think what they are seeing today is just a sneak peek of the manner in which the sea flooring will probably be affected in future.
"Because it takes years or also centuries for CO2 to fall to all-time low of the sea, nearly all the CO2 produced through human task is still at the surface," says lead writer Olivier Sulpis that is functioning on his PhD in the planet and worldly sciences division at McGill College.
"But in the future, it will get into the deep-ocean, spread out over the sea flooring, and cause much more calcite bits at the seafloor to liquify," Sulpis says.
"The rate at which CO2 is presently being produced right into the atmosphere is extremely high in Earth's background, much faster compared to at any duration since at the very least the extinction of the dinosaurs. And at a a lot much faster rate compared to the all-natural systems in the sea can deal with, so it increases stress over the degrees of sea acidification in future," he explains.OCEAN IN THE LAB
Because it's challenging and expensive to obtain dimensions in the deep-sea, the scientists produced a set of seafloor-like microenvironments in the lab, recreating abyssal bottom currents, seawater temperature level and chemistry, and sediment structures.
The experiments assisted them understand what manages the dissolution of calcite in aquatic debris and enabled them to measure exactly its dissolution rate as a function of various ecological variables. By contrasting pre-industrial and modern seafloor dissolution prices, they had the ability to extr